Where Data Tells the Story
© Voronoi 2026. All rights reserved.
In 1945, the world’s first-ever nuclear weapon was detonated at the Trinity test site in New Mexico, United States, marking the beginning of the Atomic Age.
Since then, the global nuclear stockpile has multiplied, and when geopolitical tensions rise, the idea of a nuclear apocalypse understandably causes widespread concern. But despite their catastrophically large effects, the science of how nuclear weapons work is atomically small.
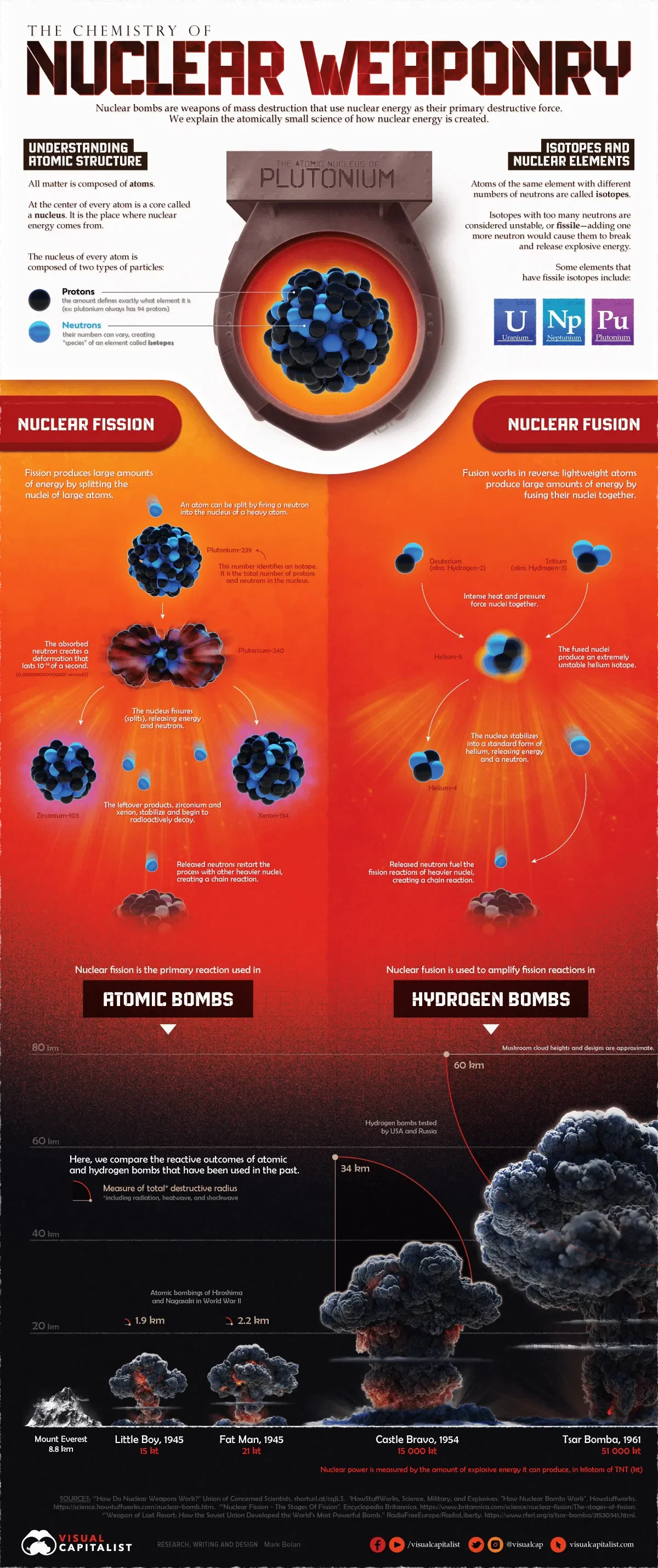
All matter is composed of atoms, which host different combinations of three particles—protons, electrons, and neutrons. Nuclear weapons work by capitalizing on the interactions of protons and neutrons to create an explosive chain reaction.
At the center of every atom is a core called the nucleus, which is composed of closely-bound protons and neutrons. While the number of protons is unique to each element in the periodic table, the number of neutrons can vary. As a result, there are multiple “species” of some elements, known as isotopes.
For example, here are some isotopes of uranium:
These isotopes can be stable or unstable. Stable isotopes have a relatively static or unchanging number of neutrons. But when a chemical element has too many neutrons, it becomes unstable or fissile. When fissile isotopes attempt to become stable, they shed excess neutrons and energy. This energy is where nuclear weapons get their explosivity from.
There are two types of nuclear weapons:
So, what exactly is the difference between fission and fusion reactions?
Nuclear fission—the process used by nuclear reactors—produces large amounts of energy by breaking apart a heavier unstable atom into two smaller atoms, starting a nuclear chain reaction.
When a neutron is fired into the nucleus of a fissile atom like uranium-235, the uranium atom splits into two smaller atoms known as “fissile fragments” in addition to more neutrons and energy. These excess neutrons can then start a self-sustaining chain reaction by hitting the nuclei of other uranium-235 atoms, resulting in an atomic explosion.
Atomic bombs use nuclear fission, though it’s important to note that a fission chain reaction requires a particular amount of a fissile material like uranium-235, known as the supercritical mass.
Hydrogen bombs use a combination of fission and fusion, with nuclear fusion amplifying a fission reaction to produce a much more powerful explosion than atomic bombs.
Fusion is essentially the opposite of fission—instead of splitting a heavier atom into smaller atoms, it works by putting together two atoms to form a third unstable atom. It’s also the same process that fuels the Sun.
Nuclear fusion mainly relies on isotopes of lighter elements, like the two isotopes of hydrogen—deuterium and tritium. When subjected to intense heat and pressure, these two atoms fuse together to form an extremely unstable helium isotope, which releases energy and neutrons. The released neutrons then fuel the fission reactions of heavier atoms like uranium-235, creating an explosive chain reaction.